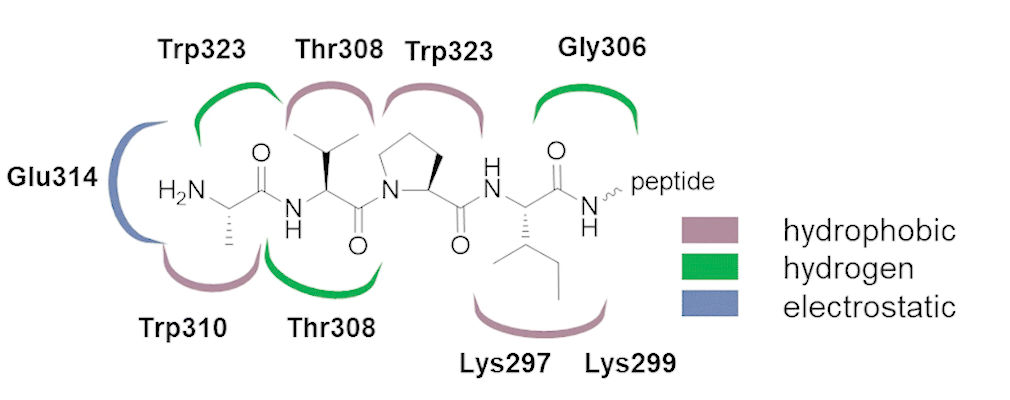
One of the strategies employed by anticancer therapies is to put the process of apoptosis back on track by blocking the interaction between inhibitor of apoptosis proteins (IAPs) and caspases. Their activity is modulated by the caspases themselves in a caspase/procaspase proteolytic cascade and by their interaction with IAPs. Caspases can be released from the inhibitory influence of IAPs by proapoptotic proteins such as secondary mitochondrial activator of caspases (Smac) that share an IAP binding motif (IBM). In this study we design and synthesize phosphorus-based peptidyl antagonists of IAPs that mimic the endogenous Smac protein, which blocks the interaction between IAPs and caspases. The ability of the obtained compounds to interact with the binding groove of the X-linked inhibitor of apoptosis protein baculovirus inhibitor of apoptosis protein repeat domain was examined by a fluorescence polarization assay, while their potential to induce autoubiquitination followed by proteasomal degradation of cellular IAP1 was examined using the breast cancer cell line. The highest potency was observed among peptides containing C-terminal phosphonic phenylalanine analogs, which displayed nanomolar Ki values. Their antiproliferative potential as well as their proapoptotic action, manifested by an increase in caspase-3 activity, was examined using various cell lines.
Read more in our article published in Investigational New Drugs